This webpage was produced as an assignment for Genetics 677, an undergraduate course at the University of Wisconsin - Madison.
Protein Motifs
Proteins are generally composed of one or more functional regions, commonly termed domains or motifs. Different combinations of domains give rise to the diverse range of proteins found in nature. The identification of domains that occur within proteins can therefore provide insights into their function. (2)
NCBI Conserved Domain Database
The Conserved Domain Datab is a protein annotation resource that consists of a collection of well-annotated multiple sequence alignment models for ancient domains and full-length proteins. (1)
CDD found 4 specific domains, 2 ABC binding cassettes (ABCC_CFTR1 in red) and 2 ABC transmembrane domains (in blue).
Pfam
The Pfam database is a large collection of protein families, each represented by multiple sequence alignments. (2)
Pfam shows 2 possible locations for the ABC Transporter Domain, which is comprised of an ABC transmembrane portion (ABC_membrane) and a Nucleotide Binding Domain (ABC_tran). This image shows the arrangement of the Pfam domains found on the CFTR protein sequence.
Pfam shows 2 possible locations for the ABC Transporter Domain, which is comprised of an ABC transmembrane portion (ABC_membrane) and a Nucleotide Binding Domain (ABC_tran). This image shows the arrangement of the Pfam domains found on the CFTR protein sequence.
SMART
SMART (a Simple Modular Architecture Research Tool) allows the identification and annotation of genetically mobile domains and the analysis of domain architectures. More than 500 domain families found in signalling, extracellular and chromatin-associated proteins are detectable. (3)
Below are the confidently predicted domains within Homo sapiens CFTR protein.There is no alternative splicing data for this protein:
AAA Domain 1
Position: 450 to 639
E-value: 3.80e-08
AAA Domain 2
Position: 1236 to 1418
E-value: 7.42e-11
Below are the confidently predicted domains within Homo sapiens CFTR protein.There is no alternative splicing data for this protein:
AAA Domain 1
Position: 450 to 639
E-value: 3.80e-08
AAA Domain 2
Position: 1236 to 1418
E-value: 7.42e-11
Analysis
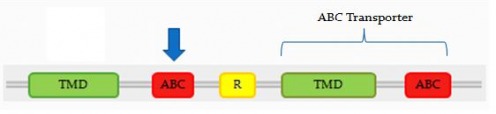
The CFTR protein is made up of 2 ABC Transporter domains and a regulatory sequence.
ABC transporters are a large family of proteins responsible for translocation of a variety of compounds across biological membranes. ABC transporters are the largest family of proteins in many completely sequenced bacteria. ABC transporters are composed of a well-conserved transmembrane domain (six transmembrane helices) and a more highly conserved nucleotide binding domain (ABC binding cassette).
The blue arrow on the below diagram indicates the location of most common CF mutation, fΔ508.Mutations are most likely to occur in the transmembrane regions as well as the first ABC binding cassette, and are least likely in the ends of the protein and the R domain. It is not surprising that the ends of the protein are least likely to have mutations, as they have no role in the protein other than to form tertiary structure, so mutations that are not severe enough to alter the overall protein conformation.
All 3 databases: CDD, Pfam, and SMART all returned the same location of the same two ABC Transporter Domains. This indicates that the CFTR is very well researched and documented in many databases.
ABC transporters are a large family of proteins responsible for translocation of a variety of compounds across biological membranes. ABC transporters are the largest family of proteins in many completely sequenced bacteria. ABC transporters are composed of a well-conserved transmembrane domain (six transmembrane helices) and a more highly conserved nucleotide binding domain (ABC binding cassette).
The blue arrow on the below diagram indicates the location of most common CF mutation, fΔ508.Mutations are most likely to occur in the transmembrane regions as well as the first ABC binding cassette, and are least likely in the ends of the protein and the R domain. It is not surprising that the ends of the protein are least likely to have mutations, as they have no role in the protein other than to form tertiary structure, so mutations that are not severe enough to alter the overall protein conformation.
All 3 databases: CDD, Pfam, and SMART all returned the same location of the same two ABC Transporter Domains. This indicates that the CFTR is very well researched and documented in many databases.
The most notable protein modification to CFTR is phosphorylation. Not until the regulatory domain is phosphorylated, can the ABC binding cassettes bind ATP. The reduction of ATP induces a conformational change, which opens the channel between the transmembrane domains that allows for the passage of chloride.
_______________
References
1. NCBI. "Conserved Domain Database". Accessed April 13, 2010. http://www.ncbi.nlm.nih.gov/Structure/cdd/wrpsb.cgi?
2. Pfam. Accessed April 22, 2010. http://pfam.sanger.ac.uk/
3. SMART. Accessed April 22, 2010. http://smart.embl-heidelberg.de/smart/set_mode.cgi?NORMAL=1
References
1. NCBI. "Conserved Domain Database". Accessed April 13, 2010. http://www.ncbi.nlm.nih.gov/Structure/cdd/wrpsb.cgi?
2. Pfam. Accessed April 22, 2010. http://pfam.sanger.ac.uk/
3. SMART. Accessed April 22, 2010. http://smart.embl-heidelberg.de/smart/set_mode.cgi?NORMAL=1
Alexandra Reynolds
[email protected]